+ General Considerations
- Testicular tumors are common and account for 4%-7% of all tumors in male dogs
- Testicular tumors broadly classified into 2 groups based on histology:
- Group I: germ cell tumors such as seminoma, embryonal carcinoma, and teratoma
- Group II: Sertoli cell tumor, interstitial cell tumor, and mixed testicular tumors
- Mixed testicular tumors may be classified separately
- Mixed germ cell-stromal tumors have a dual population of germ and Sertoli cells and account for 7% of canine testicular tumors
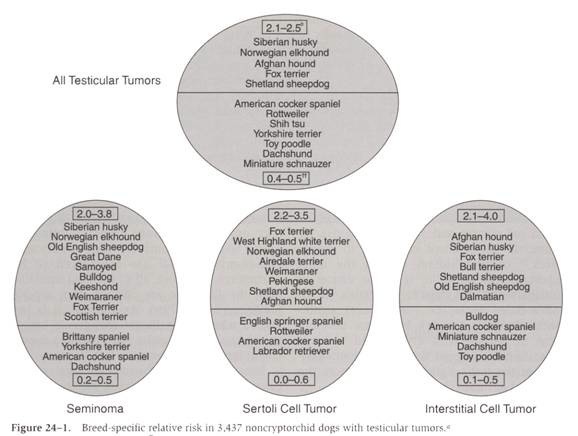
- Breed predisposition: Siberian Husky, Norwegian Elkhound, Fox Terrier, Afghan Hound, and Shetland Sheepdog
- Dachshund, Rottweiler, Shih Tzu, Yorkshire Terrier, Toy Poodle, Miniature Schnauzer, and mixed breed dogs have a significantly decreased risk of developing testicular tumors
 From: Withrow SJ & MacEwen EG (eds): Small Animal Clinical Oncology (3rd ed).
From: Withrow SJ & MacEwen EG (eds): Small Animal Clinical Oncology (3rd ed).
- 50% of dogs over 10 years have multiple tumors of different histologic types
- Testicular tumors are uncommon in dogs < 6 years
- Sertoli cell tumor and seminoma are more common with cryptorchid testicles
+ Sertoli Cell Tumor
- Sertoli cells produce estrogen, support germ cells, and form seminiferous tubule basement membrane
- Sertoli cell tumors tend to grow in expansile fashion that compress and destroy surrounding parenchyma
- Sertoli cell tumors are bilateral in 11% dogs
- 54% of Sertoli cell tumors are found in cryptorchid testicles
- 8.8-times risk of developing a Sertoli cell tumor in the cryptorchid testicle compared to descended testicle in dogs with unilateral cryptorchidism
- Testicular microenvironment influences the development of testicular tumors as, in humans, early surgical correction of cryptorchidism (i.e., orchipexy) decreases risk of testicular neoplasia
- Sertoli cell tumor in descended testicle found in younger dogs and associated with contralateral cryptorchid testicle
- Sertoli cell tumors are associated with a decreased risk of prostatic disease, circumanal gland hyperplasia, perianal tumors, and perineal hernia
- 0.6%-9.0% metastatic rate with metastatic sites including sublumbar lymph node (common), lungs, liver, spleen, adrenal glands, kidney, and pancreas
CLINICAL FEATURES
Clinical Signs
+ General Considerations
- Incidental finding at surgery or necropsy
- Scrotal or inguinal mass or enlargement
- Hypertrophic osteopathy reported in 1 dog with metastatic Sertoli cell tumor to lungs and kidney
+ Feminization Syndrome
- Feminization is rare in dogs with interstitial cell tumors and seminomas, but can occur with Sertoli cell tumors
- Feminization dependent on testicular location with feminization occurring in 16% of scrotal testes, 50% of inguinal testes, and 70% of intra-abdominal testes
- Hyperestrogenism has been implicated in the pathogenesis of feminization but this has not been proven
- Clinical signs of feminization include:
- Bone marrow hypoplasia with thrombocytopenia, hemorrhage, anemia and granulocytopenia
- Symmetrical and squamous metaplasia of the prostate resulting in cystic benign prostatic hyperplasia
- Gynecomastia and galactorrhea
- Attractiveness to other males
- Atrophy of non-neoplastic testicle due to negative feedback of estrogen on the pituitary-hypothalamus axis
- Penile atrophy with pendulous prepuce
- Bilaterally symmetrical alopecia in the genital area, inner thighs, abdomen, chest, shoulders, and thighs
- 69% mortality rate
+ Diagnosis
- Scrotal palpation
- Rectal examination, lateral abdominal radiograph, abdominal ultrasonography, or direct examination during exploratory celiotomy to assess ± biopsy the sublumbar lymph nodes
- Ultrasound examination is a sensitive and relatively specific technique for the diagnosis of testicular tumors with:
- Interstitial cell tumors appearing as a well-circumscribed mass with predominantly hypoechoic and small hyperechoic areas
- Sertoli cell tumors disrupting internal architecture with echogenic pattern varying from anechoic to mixed echogenicity
- Aspiration or biopsy are invasive, compromise testicular-blood barrier and may predispose to infertility and spermatic granuloma formation
- ± thoracic radiographs
- Histopathology following castration
+ Treatment
- Castration with resection of a large amount of the spermatic cord
- Fresh whole blood transfusion for dogs with Sertoli cell tumors if myelosuppressed with thrombocytopenia and anemia
+ Prognosis
- Castration is curative if no bone marrow hypoplasia, myelosuppression, or metastatic disease
- Mortality > 80% if severe myelosuppression
- Hematologic parameters usually improve within 2-4 weeks but can take up to 5 months to return to normal


