Author: Dr. Isabel A. Jimenez, DVM (updated May 23, 2023)
etiology /
Radiation-Induced Sarcoma /
miscellaneous /
biologic behavior /
Osteosarcoma Types /
PALLIATIVE MANAGEMENT /
LIMB AMPUTATION /
LIMB-SPARING SURGERY /
Limb-Sparing Surgery in Other Appendicular Sites /
complications /
chemotherapy /
Other Chemotherapy Protocols /
prognosis /
Novel therapies /
references /
+ ETIOLOGY
- OSA is the most common primary bone tumor in dogs (Makielski et al., 2019)
- Bone marrow mesenchymal stem cells (MSCs), also known as mesenchymal stromal cells, are thought to be progenitors of primary bone tumors.
- Mechanism may involve disruption of MSC differentiation along the osteoblastic pathway (Tang et al., 2008, Gibbs et al., 2005, Jimenez et al., 2022b; Makielski, 2019).
- Supporting this theory, conditions expected to increase bone turnover – such as bone pathology and treatment – have also been associated with OSA formation.
- Fracture-associated OSA has been documented in both humans and dogs; factors involved in the formation of fracture-associated OSA include tissue damage from original injury, cellular activity associated with fracture healing, infection, implant corrosion, or electrolysis between dissimilar metals
- Cobalt, cadmium, nickel, and cobalt-chromium-molybdenum alloys induce tumors in animal models (Huff et al., 2007)
- Polymethylmethacrylate (PMMA) and polyethylene have also been shown to induce sarcoma (Kirkpatrick et al., 2000)
- OSA formation has been associated with other bone pathologies:
- Bone infarcts (Marcellin-Little et al., 1999; Prior et al., 1985; Riser et al., 1972; Jones et al., 2020; Jimenez et al., 2022a; Jimenez et al., 2022b)
- Chronic bacterial or fungal osteomyelitis (Franchini et al., 2022; Franklin et al., 1985; Thrall, 2018)
- OCD (Holmberg et al., 2004)
- Multiple cartilaginous exostosis (Owen & Bostock, 1971)
- Bone cysts may also be a predisposing factor for OSA development, particularly if the bone is weakened to the point of pathologic fracture
- OSA development has also been associated with bone treatment:
- Internal fixation of fractures with metallic implants (Isaka et al., 2021; Gilley et al., 2017; Arthur et al., 2016; Burton et al., 2015; Stevenson et al., 1982; Pluhar, 2016; Wouda et al., 2018)
- Total hip arthroplasty (Murphy et al., 1997)
- TPLO (Selmic et al., 2018; Selmic et al., 2014)
- TTA (Dunn et al., 2012; Sharma et al., 2020)
- Radiation therapy (Gillette et al., 1990; Hosoya et al., 2008)
- In a retrospective study, history of TPLO increased the risk of proximal tibial OSA 40-fold compared to dogs without a history of TPLO (Selmic et al., 2018)
- OSA develops within the radiation field of 3.4% dogs treated with external beam radiation therapy for STS and 25% of dogs treated with intraoperative radiation therapy (Gillette et al., 1990)
- OSA has also been reported in dogs following radionuclide exposure (Gillett et al., 1985; Book et al., 1982)
+ Radiation-Induced Sarcoma
- Radiation-induced OSA is associated with high dose-fraction schemes (> 3.5-5.0 Gy/fraction) (Gillette et al., 1990)
- Criteria for the diagnosis of radiation-induced OSA (Gillette et al., 1990):
- No radiographically detectable lesion prior to radiation therapy
- OSA in the radiation-field
- Tumor type confirmed histologically
- 4-5 year asymptomatic period radiation therapy and tumor detection
- Tumor typically occurs with a low incidence at the site
+ Miscellaneous
- Rapid growth in puppies may contribute to the risk of OSA (Egenvall et al., 2007)
- Large breed dogs are predisposed to OSA, with multiple theories suggested:
- Late-closing physes are thought to be more prone to repeated physeal injury (Ehrhart et al., 2013); however, fatigue-induced injury of bone (increased microcrack density and length) caused by cyclic loading during activity was not significantly higher in the metaphysis compared to other areas (Muir & Ruaux-Mason, 2000)
- Vascular injury compromising the arterial supply to the growing end of the bone may contribute to OSA formation (Jimenez et al., 2022b)
- Pathologic fracture is a common sequela of OSA in dogs (38%), with the femur and tibia most commonly affected (Rubin et al., 2015; Jimenez et al., 2022b)
BIOLOGIC BEHAVIOR
+ Signalment
- OSA is the most common primary bone tumor in dogs (85%) and cats (70%) (Dernell et al., 2001; Thompson et al., 2017).
- OSA occurs with highest frequency in large or giant-breed dogs (77.8%) (Withrow et al., 1991; Tuohy et al., 2019; Dernell et al., 2001; Ru et al., 1998; (Edmunds et al., 2021)) with dogs <15 data-preserve-html-node="true" kg accounting for only 5% of OSA cases
- Breeds at lowest risk for OSA include the Bichon Frise, French Bulldog, and Cavalier King Charles Spaniel (Edmunds et al., 2021)
Breed predispositions:
- Rottweiler and Great Dane: >10-fold risk (Edmunds et al., 2021; McNeill et al., 2007)
- Rhodesian Ridgeback (Edmunds et al., 2021)
- Scottish Deerhound
- Irish Wolfhound (Anfinsen et al., 2011)
- Saint Bernard
- Greyhound
- Doberman
- German Shepherd Dog
- Golden Retriever
Bimodal age distribution, with 6-8% of cases in dogs <3 data-preserve-html-node="true" years of age and 80% of cases occurring in animals >7 years of age (Makielski et al., 2019)
- Age at diagnosis often 7-10 years of age (Tuohy et al., 2019)
- Sex predisposition for canine OSA has not been reliably documented, with some studies reporting a slight male predisposition (Egenvall et al., 2007; Hillers et al., 2005), others reporting a slight female predisposition (Sapierzynski & Czopowicz, 2017; Tuohy et al., 2019; Ru et al., 1998), and others no difference (Anfinsen et al., 2011).
- While spayed/neutered dogs have been reported to have a higher incidence of OSA (Cooley et al., 2002), this may be confounded by factors relating to age and adiposity (Makielski et al., 2019). Other studies varied in reported associations between reproductive status and OSA (Sapierzynski & Czopowicz, 2017; Ru et al., 1998; Hillers et al., 2005)
- Several genetic loci have been identified as risk factors for canine OSA (Karlsson et al., 2013; Sakthikumar et al., 2018), but the only variant consistently implicated in human and canine OSA cases is TP53 (Makielski et al., 2019)
Osteosarcoma Types
+ Types of osteosarcoma
- Types of OSA:
- Central (intramedullary) OSA – arise from the medullary canal
- Conventional central OSA: osteoblastic, chondroblastic, fibroblastic, mixed
- Low-grade poorly differentiated central OSA
- Small cell OSA
- Telangiectatic OSA
- Surface (peripheral) OSA – arise from the periosteum
- Parosteal (juxtacortical) OSA
- Periosteal OSA
- High-grade surface OSA
- Conventional central OSA is the most common type of OSA (95% of cases); all other types comprise the remaining 5%
- Central (intramedullary) OSA – arise from the medullary canal
+ Intramedullary osteosarcoma
- Most common type of canine OSA
- Subclassified according to predominant mesenchymal cell type:
- Osteoblastic
- Chondroblastic
- Fibroblastic
- Telangiectatic
- Poorly differentiated
- Canine OSA subclassifications have not been shown to have prognostic significance
- Subclassified according to predominant mesenchymal cell type:
- Most canine OSA is high-grade (75% grade III) (Kirpensteijn et al., 2002)
- Some studies have suggested that histologic grade affects clinical outcome in canine OSA (Kirpensteijn et al., 2002; Loukopoulos & Robinson, 2007; Cooper et al., 2002) while others have found no correlation between grade and prognosis (Nagamine et al., 2015; Schott et al., 2018)
- By the time of diagnosis, microscopic metastases are present in approximately 90% of dogs (Ehrhart et al., 2013)
- OSA lesions are typically monostotic (Thrall, 2018)
- OSA rarely crosses the joint surface, as collagenase inhibitors limit tumor cell invasion or angiogenesis across the synovium
- Differential diagnoses for lesions crossing a joint surface include synovial cell sarcoma, histiocytic sarcoma, septic arthritis, rheumatoid arthritis
+ Parosteal and Periosteal Osteosarcoma
- Parosteal and periosteal OSA arise from the periosteum (Craig et al., 2016) and are both much less common than central OSA
- Periosteal OSA:
- Originates from undifferentiated cells of the osteogenic layer of the periosteum (Craig et al., 2016; Olsen et al., 2012)
- More aggressive than parosteal OSA and may show malignant characteristics including metastasis (Craig et al., 2016; Olsen et al., 2012)
- Causes bone lysis, bone proliferation, and medullary invasion (Craig et al., 2016; Olsen et al., 2012)
- Parosteal OSA:
- Originates from the fibrous layer of the periosteum (Thompson et al., 2017)
- Typically well-differentiated, with no pleomorphism, and low mitotic index (Craig et al., 2016; Schajowicz et al., 1995; Unni et al., 1976)
- Slow-growing and tend not to metastasize (Craig et al., 2016; Olsen et al., 2012; Ahuja et al., 1977)
- Lesions are firm, smooth masses that extend from the surface of the cortical bone without cortical lysis (Thompson et al., 2017)
- Transformation to a more malignant population of cells over time has been documented (Gold et al., 2018).
+ Appendicular Sites
- OSA most commonly affects the metaphysis of long bones of the appendicular skeleton (Fletcher et al., 2013; Slayter et al., 1994, Withrow et al., 1991)
- OSA more often involves the thoracic limb (2/3 of cases) compared to the pelvic limb (1/3 of cases) (Tuohy et al., 2019)
- Most common sites for canine appendicular OSA are the metaphyses of the distal radius (23.1%), proximal humerus (18.5%), and distal femur (8.5%) (Straw et al., 1990; Ehrhart et al., 2013; Kirpensteijn et al., 2002)
- OSA commonly described to have a predilection for sites located “away from the elbow” and “towards the stifle”
- Proximal tibia (7.5%), distal tibia (8.2%), and proximal femur (5.4%) are also common sites
- Ulna is uncommon (2.6%)
- Distal humerus and proximal radius sites are rare (1%)
- Manus and pes are uncommon (1.8%)
- The most common sites of bone infarcts coincide with the most common sites of canine appendicular OSA (Jimenez et al., 2022b)
- Vascular compromise of the longer branch of the nutrient artery in these sites has been suggested as a predisposing factor for bone infarct formation in dogs.
- A subset of bone infarcts have been demonstrated to undergo neoplastic transformation to form OSA.
+ Metastasis
- Canine appendicular OSA is highly malignant; while <15% data-preserve-html-node="true" of dogs have clinical or radiographic evidence of metastasis at the time of initial diagnosis, microscopic metastasis is thought to already be present in 90% of cases (Spodnick et al., 1992; Ehrhart et al. 2013).
- The most common site of metastasis is the lung, but metastasis to regional lymph nodes, bones, liver, spleen, and skin have also been reported (Spodnick et al., 1992; Ogilvie et al., 1993; Gorman et al., 2006).
- Although there is a theoretical possibility of seeding neoplastic cells along a biopsy tract following a core needle biopsy, this risk appears to be low and should not preclude the use of these diagnostic techniques.
- The first confirmed case of seeding following FNA of OSA in a dog was reported in 2022 (Faletti et al., 2022)
- A retrospective analysis of human musculoskeletal tumors suggested that the risk of seeding following needle biopsy is low (UyBico et al., 2012)
- Care should nonetheless be made to plan the biopsy tract such that it could be surgically excised, particularly in axial locations or if limb-sparing surgery is to be attempted.
- Similar accuracy of diagnosis was present for fine needle aspiration cytology (83%; sensitivity 83.3%, specificity 80%) and core histologic biopsy (82.1%; sensitivity 72.2%, specificity 100%) when compared to the final histologic diagnosis from surgical or post-mortem samples (Sabattini et al., 2017).
- Ultrasound-guided fine needle aspiration has a sensitivity of 97% and specificity of 100% for diagnosis of osteosarcoma from appendicular bone lesions (Britt et al., 2007).
PALLIATIVE MANAGEMENT
+ Analgesia
- Appendicular OSA results in marked nociceptive and neuropathic pain, due to extensive destruction of bone and surrounding soft tissue, as well as peripheral and central sensitization (Mantyh, 2014; Monteiro et al., 2018)
- MST with palliative oral analgesics alone: 107 days (NIH National Cancer Institute)
+ External Beam Radiation Therapy
- Radiation therapy is a common local treatment modality used to alleviate pain in dogs with appendicular OSA, particularly as a component of palliative care (Mayer & Grier, 2006).
- Fractionated irradiation with curative intent appears to be ineffective (Coomer et al., 2009).
- Palliative radiation therapy reduces local inflammation, minimizes pain, slows progression of metastatic lesions, and improves quality of life in dogs with primary and metastatic lesions (Mayer & Grier, 2006)
- Palliative Radiation Protocols:
- Palliation of appendicular OSA: 2 fractions of 800 cGy, 24 hours apart (cumulative dose of 1600 cGy) (Mayer & Grier, 2006)
- Palliation of axial and appendicular OSA: four 8 Gy fractions on days 0, 7, 14, and 21 (Green et al., 2002)
- Expedited palliative radiation protocol for lytic or proliferative appendicular OSA: two 8-Gy fractions over 2 consecutive days (Knapp-Hoch et al., 2009)
- Palliation of appendicular OSA: two 10-Gy fractions over 2 consecutive days (Pagano et al., 2016).
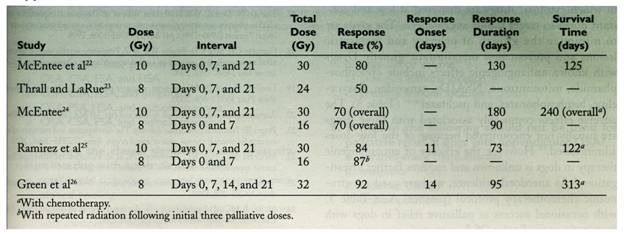
From: Liptak JM, et al: Canine appendicular osteosarcoma: diagnosis and palliative treatment. Compend Contin Educ Pract Vet, 26:172-183, 2004.
- The four-fraction protocol is effective for palliation of pain in dogs with axial and appendicular osteosarcoma, and may result in a higher response rate and longer survival time than three-fraction pallative protocols (Green et al., 2002).
- ~74-90% response rate for pain control reported in multiple studies (Green et al., 2002; Knapp-Hoch et al., 2009; Pagano et al., 2016; Ramirez et al., 1999)
- Median duration of pain relief: 67-80 days (range 12-503 days) (Pagano et al., 2016; Knapp-Hoch et al., 2009)
- Palliative radiation is generally well-tolerated in terms of side effects (Green et al., 2002; Knapp-Hoch et al., 2009; Pagano et al., 2016).
- MST with palliative radiation therapy: 122-313 days (NIH National Cancer Institute)
- Higher cumulative doses, higher intensity of treatment, and the addition of chemotherapy to palliative radiation protocols improves both the response rate and duration of response
- Dogs receiving coarse fractionation radiation therapy protocols may have a lower incidence of pathologic fracture compared to dogs receiving fine fractionation (Norquest et al., 2022).
- There is no apparent difference in outcome in dogs treated with palliative hypofractionated RT in combination with bisphosphonates (MST: 119 days) in comparison to RT alone (MST: 109 days) (Ringdahl-Maryland et al., 2022).
+ Radiopharmaceuticals
- Samarium-153-EDTMP is a medium-energy beta-particle-emitting radioisotope with localization to bone tissue; selective delivery of ionizing radiation to neoplastic lesions may limit systemic side effects (Ketring, 1987; Bryan et al., 2008)
- Internal radioisotope therapy using bone-targeting radiopharmaceutical agents may provide palliation of bone pain in patients with both primary and metastatic lesions (Lattimer et al., 1990)
- Response is better in primary bone tumors confined within the cortical margins, metastatic lesions < 2cm, and axial tumors (Lattimer et al., 1990)
- Large lesions with minimal osteoblastic reaction have poor responses as chelators bind to bone matrix (Lattimer et al., 1990)
- Complications include myelosuppression (due to proximity of bone marrow to lesion and sensitivity of proliferating marrow cells to ionizing radiation, peaks at one week and evident for 2-3 weeks), increased ALP activity without hepatocellular damage, increased bone pain after treatment (flare response due to radiation-induced endosseous edema = good prognostic sign), and cystitis (Lattimer et al., 1990).
- Complete remission was reported in 1/9 dogs treated with Sm-153-EDTMP (Milner et al., 1998).
LIMB AMPUTATION
+ General Considerations
- Limb amputation is the gold standard for the local management of primary bone tumors
- Limb amputation can be performed alone for palliative management of tumor-related pain or pathologic fracture, or in combination with adjuvant chemotherapy for curative-intent treatment
- Absolute contraindication: neurologic disease
- Relative contraindications: osteoarthritis, obesity, and large breed dogs
- Thoracic limb OSA: forequarter amputation
- Pelvic limb OSA: coxofemoral disarticulation for distal pelvic limb lesions and en bloc acetabulectomy for proximal femoral lesions
- Complications: hemorrhage, infection, and intraoperative air embolism
+ Functional Outcome
- Dogs with OSA adapt quickly following limb amputation with medium time to maximal adaptation of 4 weeks (Kirpensteijn et al., 1999)
- Kinematic analyses following hindlimb amputation demonstrate that dogs adjust to their new gait, with redistribution of weight on remaining limbs, 10 days post-procedure (Galindo-Zamora et al., 2016).
- In a survey of owners of dogs that underwent thoracic or pelvic limb amputation, 88% reported complete or near complete return to quality of life (Dickerson et al., 2015)
- 91% of owners reported no change in their dog’s attitude after amputation (Dickerson et al., 2015)
- Body condition scores and body weight at the time of amputation were negatively correlated with quality of life scores after surgery (Dickerson et al., 2015)
- In an older study, speed of adjustment was not associated with body weight, age, or thoracic or pelvic limb amputation (Kirpensteijn et al., 1999)
- Speed of adjustment is significantly quicker with a positive reaction from the family (Kirpensteijn et al., 1999)
- Change in weight-bearing and gait may lead to an increased incidence of orthopedic disease in the remaining limbs (Cole & Millis, 2017)
- Thoracic limbs normally bear 60% of body weight and contribute more to the braking phase of the gait, whereas the pelvic limbs normally bear 40% of body weight and contribute more to the propulsion phase of the gait
- Thoracic limb amputation results in:
- Remaining thoracic limb bearing 47.5% of body weight (Cole & Millis, 2017)
- Decrease in total stance time in all limbs
- Significant changes in braking and propulsion times in the remaining thoracic limb so that the pelvic limbs do not compensate for the loss of braking force and impulse
- Pelvic limb amputation results in:
- Remaining pelvic limb bearing 26% of body weight (Cole & Millis, 2017)
- No difference in stance and braking times
- Decrease in propulsive forces in the thoracic limbs
- Decrease in the time taken to reach maximal breaking force in the remaining pelvic limb
- Increase in the time taken to reach maximal breaking force in the thoracic limbs
- Forces generated through the opposite limb are greater despite the shift in the center of gravity
- Thoracic limb amputees have more difficulty in keeping balance in the early postoperative period as the ability to brake decreases resulting in loss of coordination
- Pelvic limb amputees have more difficulty in gaining speed
+ Oncologic Outcome
- MST with surgery alone: 3-5 months (Spodnick et al., 1992) / 101-175 days (NIH National Cancer Institute; Thompson & Fugent, 1992)
- 1-year survival rate 11%-21% and 2-year survival rate 0%-4%
- Dogs that lived >1 year had a median survival time of an additional 8 months (Culp et al., 2014)
- Dogs treated with amputation alone have a significantly better survival time than palliative management with analgesic drugs or radiation therapy
LIMB-SPARING SURGERY
+ General Considerations
- Indications: OSA clinically and radiographically confined to the limb and primary OSA < 50% of bone (Liptak et al., 2004; Dernell et al., 2001)
- Contraindication: pathologic fracture due to tumor-seeding into adjacent soft tissue (Liptak et al., 2004)
- The risk of local tumor contamination can be reduced by pre-operative neoadjuvant chemotherapy ± radiation therapy (Straw et al., 1996).
- Degree of bone involvement can be most accurately determined by CT (Davis et al., 2002), while regional radiographs, bone scintigraphy, and MRI may overestimate bone involvement (Davis et al., 2002; Leibman et al., 2001; Wallack et al., 2002).
- Lateromedial radiographs overestimate the degree of bone involvement by 17% and craniocaudal radiographs by 4%
- Nuclear scintigraphy overestimates the degree of bone involvement by 14%-30%
- Regional radiographs and nuclear scintigraphy are poorer predictors of bone tumor length as the bone tumor-to-total radius length increases (Leibman et al, 2001)
- CT is the most accurate technique when intramedullary fibrosis is taken into account, but CT may overestimate the degree of bone involvement by 27%
- MRI overestimates the degree of bone involvement by 3%
- Non-contrast T1-weighted images provide the best detail of intramedullary tumor extent
- Successful limb-sparing surgery in a dog with proximal femoral osteosarcoma has been reported (Liptak et al., 2005)
- Distal radius is the most amenable site for limb-sparing surgery, and the following techniques have been described:
- Allograft (Liptak et al., 2006)
- Pasteurized and irradiated autografts (Buracco et al., 2002; Morello et al., 2003)
- Vascularized autograft (Gasch et al., 2013)
- Vascularized ulnar transposition grafts (Séguin et al., 2017; Pooya et al., 2004)
- Endoprosthesis (Mitchell et al., 2016; MacDonald & Schiller et al., 2010)
- Bone transport osteogenesis (Ehrhart, 2005)
Cortical Allograft
+ Surgical Technique
- Fresh-frozen cortical allograft is thawed in a saline solution containing neomycin and penicillin
- Bone tumor is resected with 2-3 cm margins (including extensor muscles) based on preoperative imaging studies
- Proximal bone marrow is submitted for margin evaluation
- Radial carpal bone cartilage is removed to provide a flat surface to abut the cortical allograft
- Cortical allograft is cut to the appropriate size using an oscillating saw
- Cortical allograft is filled with methylmethacrylate
- Cortical allograft is plated to the proximal radius and 3rd or 4th metacarpal bone using a 3.5 mm broad DCP, 4.5 mm broad DCP, or 2.7-3.5 mm modified hybrid plate
- 2 screws are inserted into the allograft to decrease the number of screw holes and increase strength of the allograft
- Autogenous cancellous bone graft can be used to fill defect between allograft and host bone
+ Bone Cement Filling
- Polymethylmethacrylate in the medullary canal of the allograft has the following effects:
- Increases maximal cortical screw pullout force and holding strength
- Reduces screw loosening
- Reduces allograft failure
- Polymethylmethacrylate may also augment allograft strength during revascularization and resorption
- Polymethylmethacrylate has the potential to act as a reservoir for antibiotics ± chemotherapy agents
- However, bone cement may delay allograft healing at both the proximal and distal host-graft interfaces
- Papineau technique (i.e., filling the allograft with cancellous bone graft) has not been successful in improving the rate of allograft incorporation
+ OPLA-Cisplatin
- Biodegradable cisplatin-containing implant (OPLA-Pt) allows for 29-times higher concentration of cisplatin delivery to the tumor site compared to systemic administration; local delivery reduces systemic side effects (Straw et al., 1994)
- In one study, dogs treated with OPLA-Pt in the limb-sparing surgery site at the time of surgery were 53.5% less likely to develop local recurrence, although not statistically significant (p=0.071) (Withrow et al., 2004)
- Local recurrence was present in 37 dogs (46.3%); 13 in the OPLA-Pt group (32.5%) and 24 in the control group (60%)
- Median time to local recurrence: 832 days after complete resection and 256 days after incomplete resection
- No significant difference in MST between OPLA-Pt (301 days) and control (280 days)
- Administration of two doses of OPLA-Pt did not have a significant effect on disease-free interval compared to one dose (Mehl et al., 2005)
- OPLA-Pt may cause local tissue toxicity in up to 30% dogs (impaired bone formation, increased inflammation)
- Local administration of carboplatin in Poloxamer 407 achieved a local recurrence-free interval of 296 days after surgery, with survival time of 445 days from diagnosis, in a dog following limb-sparing ulnectomy (Risselada et al., 2020)
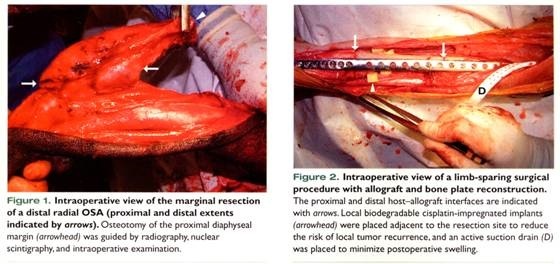 From: Liptak JM, et al: Canine appendicular osteosarcoma: curative-intent treatment. Compend Contin Educ Pract Vet, 26:186-197, 2004.
From: Liptak JM, et al: Canine appendicular osteosarcoma: curative-intent treatment. Compend Contin Educ Pract Vet, 26:186-197, 2004.
+ Pasteurized Autograft
- Pasteurized autografts have been described to reconstruct distal radial defects in several studies (Buracco et al., 2002; Morello et al., 2003)
- A segment of the distal radius derived from the tumoral bone segment was pasteurized in a sterile saline solution preheated to and maintained at 65ºC for 40 minutes to kill tumor cells; the autograft was then fixed with a plate and screws (Morello et al., 2003; Buracco et al., 2002)
- Pasteurized autografts in conjunction with adjuvant chemotherapy (cisplatin or cisplatin and doxorubicin) (Morello et al., 2003):
- Median DFI: 255 days
- MST: 324 days, with 12-month survival rate 50%, 18-month survival rate 44%, and 24-month survival rate 22%
- Complications included local tumor recurrence (15%), mild infection (31%), and implant failure (23%)
- Limb function ranged from fair in 8% (1/13 dogs) to good in 92% (12/13 dogs)
+ Distraction Osteogenesis
- Complications associated with cortical bone allografts, such as implant failure or loosening, graft rejection, and disease transmission are decreased or eliminated with the use of autogenous bone grafts (Tommasini et al., 2000, Cattaneo et al., 1992).
- Distraction osteogenesis and bone transport osteogenesis (BTO) rely on the principle that traction creates bone stress which drives osteoblast proliferation, when bone simultaneously has sufficient vascularization and functional load-bearing (Ilizarov et al., 1992)
- BTO is a subset of distraction osteogenesis in which large segmental bone defects are bridged by gradually moving the healthy bone across the defect, resulting in regeneration of bone at a rate of 1 mm per day (Aronson et al., 1997, Welch et al., 1999).
- The newly formed bone acts as highly vascularized autogenous graft that rapidly remodels into lamellar bone.
- BTO has been used to manage distal radial and tibial defects in dogs following appendicular OSA resection (Tommasini et al., 2000, Rovesti et al., 2002, Jehn et al., 2007)
- Neoadjuvant radiation therapy significantly inhibits osteogenesis whereas cisplatin and methotrexate do not affect regenerate bone formation during intercalary transport (Ehrhart et al., 2002)
- Successful application of BTO relies on strict owner compliance in distracting the apparatus 2-4 times per day. The fixator must remain in place for a long period of time, depending on the size of the defect and requires maintenance and appropriate exercise restriction, while still stimulating functional weight-bearing to promote bone repair (Tommasini et al., 2000, Jehn et al., 2007)
- Bone growth occurs at a rate of 1 mm/day (Tommasini et al., 2000)
- 83-147 days to complete transport of the intercalary segment
- For large defects, 100-147 days of distraction was reported (Tommasini et al., 2000)
- Double bone transport may be utilized to fill large defects more quickly (Rovesti et al., 2002)
- Other complications:
- Difficulty in docking the intercalary bone on to the radial carpal bone
- Necrosis of regenerate bone has been reported, but pre-operative radiation was also performed (Tommasini et al., 2000)
- Local tumor recurrence variable depending on case selection and adjuvant therapy
+ Microvascular Transfer
- Microvascular transfer of the mid-diaphysis of the ulna has been described based on the common interosseous vein and artery with a cuff of abductor pollicis longus and deep digital flexor muscles (Walsh et al., 2000)
- Proximal ulnar graft maintains both the nutrient artery and periosteal circulation, whereas distal ulnar grafts only preserve periosteal circulation
- Successful transfer occurred despite lack of preservation of the nutrient artery and vein
- Vascularized autogenous bone is resistant to infection and actively contributes to bone healing
- Other sites for autogenous cortical bone harvest include:
- Proximal ulna (based on common interosseous pedicle)
- Distal ulna (based on caudal interosseous pedicle)
- Medial tibia (based on medial saphenous pedicle)
- Trapezius myo-osseous flap (based on the prescapular branch of the superficial cervical vessels)
Roll-Over Technique
+ General Considerations
- In the ulnar rollover transposition (URT) technique, a distal ulna osteotomy is performed and the segment is rolled into the radial defect and secured with a bone plate and screws (Séguin et al., 2003; Séguin et al., 2017)
- Requires vascular preservation of the caudal interosseous artery and vein and a muscular cuff of the deep digital flexor, abductor pollicis longus, and pronator quadratus muscles to maintain viability of the transplanted bone (Séguin et al., 2003)
- MST: 277 days (Séguin et al., 2017)
- Median DFI: 245 days (Séguin et al., 2017)
- Contraindications: ulnar involvement or involvement of the caudal muscles and area surrounding the host vessels
+ Surgical Technique
- Abductor pollicis longus and pronator quadratus attachments to the periosteum of the ulna are preserved during resection of the distal radius
- Ulna is osteotomized between the articular surface and metaphyseal area which allows the radius and styloid process to be resected en bloc
- 2nd ulna osteotomy is performed 1-2 mm distal to radial osteotomy
- Preservation of the caudal interosseous artery and vein during radial dissection and ulna osteotomy is important
- Pronator quadratus remains intact proximally but resected distally
- Abductor pollicis longus is sectioned both proximally and distally
- Ulnar head of the deep digital flexor remains intact both proximally and distally
- Ulnar autograft is rolled 90º into the radial defect so that surface of bone exposed between attachments of abductor pollicis longus and ulnar head of the deep digital flexor is in the same plane as the cranial surface of the radius
+ Functional and Oncologic Outcome
- Caudal interosseous artery and vein can tolerate 90º rotation with minimal risk of disturbing vascular flow
- Caudal interosseous artery patency was preserved in 83% (10/12) of latex injected limbs and patent vessels were present in the medullary cavity of the ulna autograft in 100% (6/6) of barium sulfate injected limbs (Séguin et al., 2003)
- Limbs reconstructed with cortical allografts are biomechanically superior to ulnar transposition in axial loading, with:
- 68% greater stiffness
- 62% greater yield load
- 60% greater maximum load
- 30% greater maximum energy
- 28% greater post-yield energy
- However, mean yield loads are > 3 times peak vertical ground reaction forces for a normal dog at the trot
- Limbs reconstructed with an ulnar roll-over fail because of cranial plate bending (Séguin et al., 2003; Séguin et al., 2017)
- Ulnar transposition results in limb shortening by 6%-10% in anatomic studies and 11%-24% in 3 clinical cases (Séguin et al., 2003)
- Biomechanical complication rate was high, but limb function was generally good (Séguin et al., 2003; Séguin et al., 2017)
- Local recurrence was not increased compared to other limb-sparing techniques, with appropriate case selection (Séguin et al., 2017)
+ Endoprosthesis
- Endoprosthesis is a surgical steel spacer which is commonly used in human limb-sparing surgery
- In dogs, cortical allografts rarely become fully incorporated, and thus functionally act as spacers (Liptak et al., 2006a)
- Limbs reconstructed with an endoprosthesis are biomechanically superior to cortical allograft-reconstructed limbs in axial loading, with (depending on whether the distal ulna is resected or preserved) (Liptak et al., 2006b):
- 47%-85% greater yield load
- 41%-61% greater maximum load
- 64%-133% greater yield energy
- 26%-33% greater stiffness
- There were no significant differences in surgical or oncologic outcome for distal radial OSA managed with cortical allografts versus endoprostheses (Liptak et al., 2006a)
- Infection rate: 50-60%
- Construct failure rate: 40%
- Limb function: good to excellent in 80% of dogs
- Local tumor recurrence rate: 20%
- Metastatic rate: 60%
- Limb function was not associated with postoperative infection, construct failure, or local tumor recurrence (Liptak et al., 2006a)
- MST was significantly higher in dogs with construct failure (685 days vs. 332 days) and infection (685 days vs. 289 days) (Liptak et al., 2006a)
Limb-Sparing Surgery in Other Appendicular Sites
+ Humerus
- Limb-sparing of the proximal humerus has been described (Kuntz et al., 1998):
- Craniolateral approach
- Radial nerve and cephalic vein are preserved
- Shoulder joint disarticulated after transecting the transverse band of the intertubercular groove and retracting the biceps brachii muscle
- Tumor is resected with margin of normal deltoid, infraspinatus, teres minor, and brachialis muscles
- Shoulder arthrodesis is performed with an allograft 2 cm shorter than the resected bone to prevent a decrease in the range of motion of the elbow
- 2nd plate at the distal allograft-host interface
- Pectoral muscles reattached to allograft with non-absorbable sutures
- Mechanical failure with spiral fracture of the distal humerus is a common postoperative complication due to the large lever arm and stress concentration caused by arthrodesis; double plating is recommended to reduce the risk of this complication
- Completeness of resection is a positive prognostic indicator for both local tumor recurrence and distant metastases
- Good-to-excellent outcome in only 12% of 17 dogs with proximal humeral OSA
- “Limb salvage surgery for OS of the proximal humerus in dogs cannot be recommended until improvement in functional outcome and reduction in postoperative complications can be achieved” (Kuntz et al., 1998)
+ Ulna
- Ulnectomy can be performed below the interosseous ligament without reconstruction and good functional results
- Styloid process resection, without surgical stabilization of the carpus via pancarpal arthrodesis, can result in adequate limb function and minimal lateral instability, as long as resection is performed close to the carpal bone (Sivacolundhu et al., 2013; Amsellem et al., 2017)
- Ulnectomy above the interosseous ligament causes radioulnar instability and elbow joint incongruity
- Telangiectatic and telangiectatic-mixed subtypes were negative prognostic indicators for DFI and survival time in dogs with ulnar OSA (Sivacolundhu et al., 2013)
+ Digit
- Digit OSA is rare (Gamblin et al., 1995; Tuohy et al., 2019) and comprises <2% data-preserve-html-node="true" of all digit masses in dogs (Marino et al., 1995)
- Digit, metacarpal, and metatarsal OSA appear to have a longer MST compared to OSA of other appendicular locations (Tuohy et al., 2019)
- A review of canine digit OSA reported an overall MST of 687 days (Tuohy et al., 2019)
- No apparent difference in MST was noted between dogs that received surgery alone vs. surgery with adjuvant chemotherapy, but the number of cases analyzed was not sufficient for statistical power (Tuohy et al., 2019)
- A high rate of metastasis (~46%) was observed (Tuohy et al., 2019)
- Digit amputation is a form of limb-sparing surgery
- Inclusion of the metatarsal / metacarpal bone in the amputation depends on tumor site (Tuohy et al., 2019; Hall & Mason, 2019)
- If the metatarsal / metacarpal bone is included, proximal ostectomy or disarticulation is recommended to achieve adequate proximal margins
- Lameness can occur with ostectomy at the level of the mid-to-distal metacarpus or metatarsus
- Digit amputation resulted in adequate local tumor control in 9/11 cases (Tuohy et al., 2019)
- Partial or complete limb amputation may be more beneficial in patients with tumors in metacarpal/metatarsal III or IV to improve margins and decrease the risk of local recurrence (Tuohy et al., 2019)
+ Postoperative Management
Exercise limited for 3-4 weeks but encouraged to reduce foot swelling and flexural contracture of the digits
Complications
+ Infection
- Infection is the most significant complication of limb-sparing surgery (Dernell et al., 2001; Straw et al., 1996; LaRue et al., 1989; Morello et al., 2001; Kirpensteijn et al., 1998; Dernell et al., 1998, Liptak et al., 2004)
- Infection occurs in over 40% of cases, with approximately 2/3 of infections diagnosed ≥6 months after surgery (LaRue et al., 1989; Morello et al., 2001; Kirpensteijn et al., 1998; Dernell et al., 1998)
- Numerous bacterial organisms have been cultured, with monomicrobial and polymicrobial infections occurring in approximately 50% dogs (Dernell et al., 1998)
- Cause of infection is unknown but may include:
- Immunosuppression caused by the tumor or treatment (i.e., anesthesia, surgery, and chemotherapy)
- Extensive soft tissue resection with vascular compromise to a poorly perfused site
- Limited soft tissue coverage
- Implantation of orthopedic implants
- Implantation of non-vascularized and possibly immunogenic cortical bone
- Administration of local and systemic chemotherapy
- Infection significantly increases survival time compared to dogs treated with limb amputation or non-infected limb-sparing surgery, possibly due to activation of immune effector cells and response to cytokines (i.e., IL-1 and TNF) (Liptak et al., 2001)
- Treatment of SSI at the site of limb-sparing surgery may be managed with antibiotics, wound lavage, and wet-to-dry bandages (Dernell et al., 2001, Dernell et al., 1998).
- If infection is resistant to treatment, antibiotic-impregnated polymethylmethacrylate beads can provide local antibiotic therapy (Dernell et al., 1998).
- Limb amputation is a salvage procedure employed in 16% (Wustefeld-Janssens et al., 2020)
+ Local Recurrence
- Local tumor recurrence can be caused by residual microscopic tumor burden in adjacent soft tissue (common) or incomplete resection (uncommon, as tumor recurrence rarely occurs at allograft-host interface)
- Local tumor control following limb-sparing surgery is associated with percent tumor necrosis; tumor necrosis greater than 80% was statistically associated with lack of recurrence (LaRue et al., 1989)
- Neoadjuvant chemotherapy and radiation therapy is used in humans prior to limb-sparing surgery to reduce the risk of local tumor recurrence
+ Orthopedic Failure
- 11%-60% dogs have implant failure following limb-sparing surgery (Straw et al., 1996; Morello et al., 2001; Kirpensteijn et al., 1998)
- Implant failure is often a sequela to infection, but other causes include aseptic screw loosening in the allograft during revascularization and resorption phases
- Polymethylmethacrylate (PMMA) bone cement in the medullary canal of the allograft can be employed to reduce the rate of failure and complications, but does not completely eliminate the occurrence of infection or aseptic loosening (Phull et al., 2021)
- Limbs reconstructed with an ulnar roll-over technique (URT) can fail because of cranial plate bending (Séguin et al., 2003; Séguin et al., 2017)
- Limbs reconstructed with a cortical allograft fail because of either caudal plate bending (50%) or fracture of the 3rd metacarpal bone at the distal aspect of the plate (50%) (Liptak et al., 2006)
- 54% of non-cemented and 60% cemented allografts fail distal to the antebrachiocarpal joint
- Metacarpal bone fracture was significantly more likely if the plate covered < 80% of the metacarpal bone
+ Other Complications
- Complications reported in humans with limb-sparing surgery include allograft resorption (30%), allograft fracture (19%-28%), and nonunion of the allograft-host bone interface (10%-17%)
- Nonunion occurs in 18% of dogs with limb-sparing surgery using cemented allografts
CHEMOTHERAPY
+ General Considerations
- Amputation of the affected limb followed by adjuvant chemotherapy is the current standard of care for canine appendicular OSA, regardless of histopathologic grade.
- Adjuvant chemotherapy significantly improves survival time; MST with adjuvant chemotherapy is 8-12 months, compared to 3-5 months with surgery alone (Mauldin et al., 1988; Spodnick et al., 1992; Berg et al., 1997)
- Carboplatin is associated with fewer gastrointestinal signs and nephrotoxicity compared to cisplatin in a rat model (Yasumasu et al., 1992)
- In a randomized, phase III clinical trial in 50 dogs with appendicular OSA, administration of carboplatin alone led to a higher disease-free interval (425 days) compared to alternating carboplatin with doxorubicin (135 days) (Skorupski et al., 2016)
- Chemotherapy timing is an important consideration in humans with OSA, as neoadjuvant chemotherapy significantly increases survival time and provides an indication of tumor responsiveness to chemotherapy (Imran et al., 2009; Meyers et al., 1992)
- In dogs, adjuvant chemotherapy is often started 2 weeks after amputation, coinciding with timing of skin healing
- In a recent study, initiation of adjuvant chemotherapy within 5 days after limb amputation was associated with significant and clinically relevant survival benefit for dogs with appendicular OSA without distant metastases (Marconato et al., 2021)
- A prior study showed no difference in survival when chemotherapy was started 2 days vs. 10 days after amputation (Berg et al., 1997)
- Grade 4 toxicities were significantly more likely when chemotherapy is started 2 days compared to 10 days after surgery (35% v 12%) (Berg et al., 1997)
- Hematology ± urinalysis and renal profile should be performed prior to chemotherapy administration
- Chemotherapy is considered safe to administer when:
- Leukocytes > 3,000/μL
- Platelets > 100,000/μL
- BUN and creatinine within the reference range
- USG > 1.030 with no proteinuria or cast
+ Cisplatin
- Alkylating agent which binds DNA and produces cross-linkage, thus inhibiting DNA transcription
- Dose: 70 mg/m2 IV q21d for 2-6 treatments (Straw et al., 1991; Ogilvie et al., 1993)
- Higher cumulative doses and more than 3 treatments have better prognosis
- Aggressive isotonic saline diuresis is required to prevent nephrotoxicosis
- Other adverse effects include nausea and myelosuppression
- Inappetence and decreased activity level is common in the first few days after administration
- Amifostine reduces nephrotoxicity in humans and allows higher doses to be administered (Hartmann et al., 2000)
+ Carboplatin
- 2nd generation platinum compound with less nephrotoxicity
- Dose: 300 mg/m2 IV q21d for 4-6 treatments (Selmic et al., 2014)
+ Loboplatin
- 3rd generation platinum compound
- Dose: 35 mg/m2 IV q21d for 4 treatments (Kirpensteijn et al., 2002)
+ Doxorubicin
- Antibiotic that intercalates DNA and impairs DNA, RNA, and protein synthesis
- Dose: 30 mg/m2 IV q14d or q21d for 5 cycles (Selmic et al., 2014)
- Baseline echocardiogram should be obtained; decreased cardiac contractility should be a contraindication for the use of doxorubicin
- Cardiac screening is particularly warranted in breeds predisposed to congenital cardiomyopathy (e.g. Doberman, Boxer, Great Dane) and these breeds are at a higher risk for development of DCM (15.4%) in comparison to low-risk breeds (3.0%) (Hallman et al., 2019)
- Administration of doxorubicin over a 1-hour time period resulted in fewer ECG abnormalities compared to dogs that received the drug over 10 minutes (Gillings et al., 2009)
- Complications:
- Extravasation causes severe tissue necrosis
- Myelosuppression
- Transient hemorrhagic colitis
- Cardiac toxicity (if cumulative dose exceeds 180-240 mg/m2 (Hallman et al., 2019), or in predisposed breeds)
Other Chemotherapy Protocols
+ Doxorubicin and Cisplatin
- 15 mg/m2 doxorubicin and 50 mg/m2 cisplatin on consecutive days q21d for 4 treatments (Chun et al., 2000)
- 25 mg/m2 doxorubicin and 60 mg/m2 cisplatin on consecutive days q21d for 3 treatments (DeRegis et al., 2003)
- Mechanisms of action of doxorubicin and platinum drugs are different and hence can be used in combination
- Grade 4 toxicities were significantly more likely in dogs treated with doxorubicin at 25 mg/m2 vs. 12.5 mg/m2 (67% v 25%) (DeRegis et al., 2003)
+ Doxorubicin and Carboplatin
- Alternating carboplatin 300 mg/m2 IV and doxorubicin 30 mg/m2 IV q21d for 3 cycles (Selmic et al., 2014)
- Carboplatin 175 mg/m2 and doxorubicin 15 mg/m2 q21d for a maximum of 4 cycles (Bailey et al., 2003)
+ OPLA-Cisplatin
- Dose: 80 mg/m 2 implanted at amputation and 30 days postoperatively
- Median DFI 256 days
- MST 278 days, with 12-month survival rate 41%
- DFI and MST statistically similar to amputation and 2 intravenous doses of cisplatin
+ Metronomic Chemotherapy
- Metronomic chemotherapy (MC) is the continuous / frequent delivery of low and minimally toxic doses of chemotherapy agents
- Goal: target tumor angiogenesis and neovascularization, minimize the growth of primary and metastatic lesions, and prevent the development of new metastases
- Drugs with known antiangiogenic effects include cyclophosphamide, mitoxantrone, NSAIDs (e.g. piroxicam), tamoxifen, doxycycline, bisphosphonates, and paclitaxel
- Tetracycline antibiotics, such as doxycycline and minocycline, act as MMP-1 inhibitors which inhibits the growth of OSA cells in a time- and dose-dependent manner
- Adverse effects commonly seen with these drugs are usually not encountered due to the low doses administered
- Metronomic chemotherapy with piroxicam and cyclophosphamide can be safely administered in combination with maximal-tolerated doses of carboplatin +/- doxorubicin (Bracha et al., 2014)
- Lomustine (60 mg/m2) was well-tolerated in dogs with metastatic OSA (Tripp et al., 2011)
PROGNOSIS
+ General Considerations
- Prognostic factors include:
- Body weight: dogs < 40 kg have significantly longer DFI and MST (Dernell et al., 2001)
- Age: dogs < 5 years and > 10 years (Spodnick et al., 1992)
- Tumor site: proximal humerus OSA has significantly shorter DFI and MST, possibly due to larger tumor volume before diagnosis (Boerman et al., 2012)
- Tumor volume: large tumors have a poor prognosis
- Elevated Serum alkaline phosphatase (SALP) is a significant negative prognostic indicator for canine OSA (Boerman et al., 2012)
- Percent tumor necrosis is strongly predictive for local tumor control, but there was no correlation between percent tumor necrosis and time to metastasis (Powers et al., 1991).
- Whether histologic grade affects clinical outcome in canine OSA is controversial (Kirpensteijn et al., 2002; Loukopoulos & Robinson, 2007; Cooper et al., 2002; Nagamine et al., 2015; Schott et al., 2018)
- Infection in limb-sparing surgery is a positive prognostic indicator (Culp et al., 2014; Liptak et al., 2006; Lascelles et al., 2005)
- Dogs that survived for >1 year following initial diagnosis of OSA had a subsequent MST of 8 months (Culp et al., 2014).
+ Alkaline Phosphatase
- Bone-specific and total ALP are prognostic in dogs with appendicular OSA (Erhart et al., 1998; Garzotto et al., 2000)
- Preoperative total serum ALP > 110 U/L
- Median DFI 170 days v 366 days
- MST 177 days v 495 days (or 5.5 months vs 12.5 months)
- Preoperative bone-specific ALP > 23 U/L
- Median DFI 147 days v 431 days
- MST 218 days v 546 days (or 9.5 months vs 16.6 months)
- Preoperative total serum ALP > 110 U/L
- Failure of BALP activity to decrease after surgery was a negative prognostic indicator, with shorter survival and DFI (Erhart et al., 1998)
- Serum BALP activity correlates with absolute primary tumor size in dogs without microscopic metastasis (Sternberg et al., 2013)
- Increase in serum BALP coincided with development of macroscopic metastasis, and OSA in metastatic lesions retained BALP expression (Sternberg et al., 2013)
- Increased pre-treatment BALP was associated with a poorer clinical prognosis, likely due to larger tumor burden at the time of diagnosis (Sternberg et al., 2013)
- In vitro BALP expression did not correlate with in vivo aggressive characteristics of canine osteoblasts (Sternberg et al., 2013)
- For each 100 U/L increase in either total or bone-specific ALP, the risk of tumor-related death increased by 25% (Garzotto et al., 2000)
- Intratumoral ALP predicts eventual development of pulmonary metastases (Levine et al., 1979)
+ Tumor Necrosis
- Percent tumor necrosis is strongly predictive for local tumor control (Powers et al., 1991)
- 87.5% of dogs with >80% tumor necrosis had local control
- 27.6% of dogs with <79% data-preserve-html-node="true" tumor necrosis had local control
- There was no correlation between percent tumor necrosis and time to metastasis (Powers et al., 1991)
- Percent tumor necrosis in untreated OSA was 26.8% (Powers et al., 1991)
- Percent tumor necrosis in primary and metastatic lesions following neoadjuvant chemotherapy correlates with response to therapy, metastatic disease, and local tumor recurrence in humans (Hanafy et al., 2018)
90% tumor necrosis is associated with a better prognosis in humans (Richardson et al., 2022)
- Chemotherapy protocol is typically changed if tumor necrosis is poor as indicates tumor is refractory to neoadjuvant chemotherapy protocol
+ Infection in Limb-Sparing Surgery
- Dogs that developed a surgical-site infection following limb-sparing surgery had significantly improved median disease-free interval and median survival time compared to dogs that did not develop surgical-site infection (Culp et al., 2014; Liptak et al., 2006; Lascelles et al., 2005)
- MST with SSI: 685 days (Liptak et al., 2006)
- MST without SSI: 289 days (Liptak et al., 2006)
- MST after 1 year, with SSI: 180 days (Culp et al., 2014)
- MST after 1 year, without SSI: 28 days (Culp et al., 2014)
- Underlying mechanisms are unknown and should be further investigated, but similar findings have been reported in human OSA cases (Jeys et al., 2007)
- The extended survival associated with SSI after limb-sparing surgery does not appear to be present after amputation (Hans et al., 2018).
NOVEL THERAPIES FOR CANINE APPENDICULAR OSA
+ General Considerations
- Microwave ablation has been investigated as a method to induce tumor necrosis in dogs with distal radial OSA; tumor necrosis was variable but no immediate postablation complications were observed (Salyer et al., 2020)
- Histotripsy ablation has been investigated as a modality for targeted tissue destruction in excised canine OSA (Arnold et al., 2021)
- Autologous activated T-cell therapy has been described in 23 dogs with appendicular OSA; disease free interval was 213 days and MST was 415 days (Flesner et al., 2020).
- A recombinant vaccine (ADXS31-164) utilizing a Listeria monocytogenes vector showed promising results in a phase I dose escalation clinical trial, in 18 client-owned dogs that had previously completed amputation and adjuvant chemotherapy (Mason et al., 2016)
- For dogs treated with the vaccine, the median disease-free interval was 956 days compared to 123-257 days for amputation and carboplatin alone.
- Incidence of metastases was also lower in the vaccine-treated dogs compared to historical data.
- A low rate of Listeria-positive infections following vaccine administration have been described (Mussner et al., 2021; Mussner et al., 2019)
- A recombinant vaccine expressing IL-2, derived from engineered Salmonella enterica serovar Typhimurium, was evaluated in a phase I clinical trial in 19 client-owned dogs, in combination with amputation and adjuvant doxorubicin (Fritz et al., 2016)
- The dose was given at day 0, amputation was performed at day 10, and chemotherapy was initiated 2 weeks afterwards
- The utility of Salmonella spp. as a vector is due to their innate distribution to hypoxic or anaerobic environments, potentially resulting in targeting of IL-2 to the tumor microenvironment, leading to immune-mediated cytotoxicity of Salmonella-infected OSA cells
- Median DFI was 199d
- A replication-deficient adenoviral vector (Ad) was used to deliver Fas Ligand (FasL) with the goal of induction of apoptosis of OSA cells (Modiano et al., 2012)
- A Phase I clinical trial was performed in 56 dogs with appendicular OSA, in which the Ad-FasL was delivered 10 days prior to initiation of standard of care.
- Ad-FasL resulted in significantly higher survival (98 weeks compared to 37 weeks in historical controls) in a subset of dogs
- Ad-FasL may be most effective in tumors expressing low levels of FasL
- Stereotactic body radiation therapy (SBRT) resulted in improvement of lameness in 84% of dogs, for a median of 6 months duration, and histopathologic evidence of local disease control, and requires further research as a non-surgical limb sparing treatment option for canine appendicular OSA (Martin et al., 2021)
- Dose: three fractions of 36 Gy each (Martin et al., 2021)
- Over 1/3 of irradiated sites in this study resulted in a fracture; post-irradiation changes to bone result in increased fracture risk (Martin et al., 2021)
- Skin side effects of SBRT correlate significantly with dose (Martin et al., 2021).
- Concurrent administration of carboplatin and Palladia (toceranib), a receptor tyrosine kinase inhibitor approved by the FDA for treatment of mast cell tumors, did not improve outcomes in dogs with OSA (Poon et al., 2020).
- The mTOR pathway has been implicated in OSA metastasis.
- Rapamycin, an mTOR inhibitor, has been evaluated in dogs with appendicular OSA, with a safe pharmacological oral dose determined; however, further research is necessary to determine clinical effects (Paolini et al., 2010)
- Adjuvant sirolimus, an mTOR inhibitor, did not result in significant differences in DFI or MST compared to standard of care (LeBlanc et al., 2021)
- A caninized monoclonal antibody against nerve growth factor, NV-01, has been shown to ameliorate lameness in comparison to meloxicam, in canine model of footpad inflammation (Gearing et al., 2013), but has not been evaluated in dogs with OSA
- Canine OSA cells express NGF ligands (Shor et al., 2015), therefore NV-01 may be a potential therapeutic candidate for analgesia in dogs with OSA (Enomoto et al., 2019)
- MRI-guided cryoablation has been utilized to treat vertebral body OSA in dogs and is a topic of ongoing research (Krimins et al., 2017)
References
+ Listed in alphabetical order
Ahuja SC, Villacin AB, Smith J, et al. 1977. Juxtacortical (parosteal) osteogenic sarcoma: histological grading and prognosis. J Bone Joint Surg Am. 59:632–647.
Amsellem PM, Young AN, Muirhead TL, et al. 2017. Effect of distal ulnar ostectomy on carpal joint stability during weight bearing in the dog. Vet Surg. 46:1154-1160.
Anfinsen KP, Grotmol T, Bruland OS, et al. 2011. Breed-specific incidence rates of canine primary bone tumors: A population based survey of dogs in Norway. Can J Vet Res. 75:209–215.
Aronson J. 1997. Limb-lengthening, skeletal reconstruction, and bone transport with the Ilizarov method. J Bone Joint Surg Am. 79:1243-1258.
Arnold L, Hendricks-Wenger A, Coutermarsh-Ott S, et al. 2021. Histotripsy Ablation of Bone Tumors: Feasibility Study in Excised Canine Osteosarcoma Tumors. Ultrasound Med Biol. 47:3435-3446.
Arthur EG, Arthur GL, Keeler MR, et al. 2016. Risk of osteosarcoma in dogs after open fracture fixation. Vet Surg. 45:30–35.
Bailey D, Erb H, Williams L, et al. 2003. Carboplatin and doxorubicin combination chemotherapy for the treatment of appendicular osteosarcoma in the dog. J Vet Intern Med. 17:199-205.
Berg J, Gebhardt MC, Rand WM. 1997. Effect of timing of postoperative chemotherapy on survival of dogs with osteosarcoma. Cancer 79:1343–1350.
Bracha S, Walshaw R, Danton T, et al. 2014. Evaluation of toxicities from combined metronomic and maximal-tolerated dose chemotherapy in dogs with osteosarcoma. J Small Anim Pract. 55:369-74.
Britt T, Clifford C, Barger A, et al. 2007. Diagnosing appendicular osteosarcoma with ultrasound-guided fine-needle aspiration: 36 cases. J Small Anim Pract. 48:145-150.
Boerman I, Selvarajah GT, Nielen M,et al. 2012. Prognostic factors in canine appendicular osteosarcoma - a meta-analysis. BMC Vet Res. 8:56.
Book SA, Spangler WL, Swartz LA. 1982. Effects of lifetime ingestion of 90Sr in beagle dogs. Radiat Res 90:244-251.
Bryan JN, Bommarito D, Kim DY, et al. 2008. Comparison of systemic toxicities of 177-Lu-DOTMP and 153-Sm-EDTMP administered intravenously at equivalent skeletal doses to normal dogs. J Nuc Med Tech. 37:46-52.
Buracco P, Morello E, Martano M, et al. 2002. Pasteurized tumoral autograft as a novel procedure for limb-sparing in the dog: A clinical report. Vet Surg. 31:525–532.
Burton AG, Johnson EG, Vernau W, et al. 2015. Implant-associated neoplasia in dogs: 16 cases (1983–2013) J Am Vet Med Assoc 247:778-785.
Cattaneo R, Catagni M, Johnson EE. 1992. The treatment of infected nonunions and segmental defects of the tibia by the methods of Ilizarov. Clin Orthop 280:143-152.
Cole GL, Millis D. 2017. The effect of limb amputation on standing weight distribution in the remaining three limbs in dogs. Vet Comp Orthop Traumatol.30:59-61.
Chun R, Kurzman ID, Couto CG, et al. 2000. Cisplatin and doxorubicin combination chemotherapy for the treatment of canine osteosarcoma: a pilot study. J Vet Intern Med. 14:495-8.
Cooley DM, Beranek BC, Schlittler DL, et al. 2002. Endogenous gonadal hormone exposure and bone sarcoma risk. Cancer Epidemiol Biomark Prev 11:1434–1440.
Cooper S, Black AP, Smith BA, et al. 2002. Low grade osteosarcoma in a dog. Aust Vet Pract 32:104-111.
Cooper A, Farese F, Milner R, et al. 2009. Radiation therapy for canine appendicular osteosarcoma. Vet Comp Onc. 7:15-27.
Craig LE, Dittmer KE, Thompson KG. 2016. Bone and joints. In: Maxie MG, ed. Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. 6th ed. St. Louis, MO: Elsevier; 17–163.
Culp WTN, Olea-Popelka F, Sefton J, et al. 2014. Evaluation of outcome and prognostic factors for dogs living greater than one year after diagnosis of osteosarcoma: 90 cases (1997-2008). J Am Vet Med Assoc. 245:1141-1146.
Davis GJ, Kapatkin AS, Craig LE, et al. 2002. Comparison of radiography, com- puted tomography, and magnetic resonance imaging for evaluation of appendicular osteosarcoma in dogs. J Am Vet Med Assoc. 220:1171–1176.
DeRegis CJ, Moore AS, Rand WM, et al. 2003. Cisplatin and doxorubicin toxicosis in dogs with osteosarcoma. J Vet Intern Med. 17:668-73.
Dernell WS, Straw RC, Withrow SJ. 2001. Tumors of the skeletal system, In Withrow SJ, MacEwen EG (eds): Small Animal Clinical Oncology. Philadelphia, WB Saunders; 378-417.
Dernell WS, Withrow SJ, Straw RC, et al. 1998. Clinical response to antibiotic impregnated polymethyl methacrylate bead implantation in dogs with severe infections after limb sparing and allograft replacement—18 cases (1994-1996). Vet Comp Orthop Traumatol 11:94-99.
Dickerson VM, Coleman KD, Ogawa M, et al. 2015. Outcomes of dogs undergoing limb amputation, owner satisfaction with limb amputation procedures, and owner perceptions regarding postsurgical adaptation: 64 cases (2005-2012). J Am Vet Med Assoc. 247:786-92.
Dunn AL, Buffa E, Hanshaw DM, et al. 2012. Osteosarcoma at the site of titanium orthopedic implants in a dog. Aus Vet J. 90:39-43.
Edmunds GL, Smalley MJ, Beck S, et al. 2021. Dog breeds and body conformations with predisposition to osteosarcoma in the UK: a case-control study. Canine Med Genet. 8:2.
Egenvall A, Nødtvedt A, von Euler H. 2007. Bone tumors in a population of 400 000 insured Swedish dogs up to 10 y of age: incidence and survival. Can J Vet Res. 71:292–9.
Ehrhart N, Eurell JA, Tommasini M, et al. 2002. Effect of cisplatin on bone transport osteogenesis in dogs. Am J Vet Res. 63:703-711.
Ehrhart NP, Ryan SD, Fan TM. 2013. Chapter 24: Tumors of the Skeletal System, p 463-503. In: Vail DM, Thamm D, Liptak J, editors. Withrow and MacEwen’s Small Animal Clinical Oncology. Philadelphia (PA): Saunders.
Ehrhart N, et al. 1998. Prognostic importance of alkaline phosphatase activity in serum from dogs with appendicular osteosarcoma: 75 cases (1990-1996). J Am Vet Med Assoc. 213:1002-1006.
Ehrhart N. 2005. Longitudinal bone transport for treatment of primary bone tumors in dogs: technique description and outcome in 9 dogs. Vet Surg. 34:24-34.
Enomoto M, Mantyh PW, Murrell J, et al. 2019. Anti-nerve growth factor monoclonal antibodies for the control of pain in dogs and cats. Vet Rec. 184:23.
Faletti T, Seguin B, Selmic LE, et al. 2022. Potential Seeding from Fine-Needle Aspiration of an Axial Osteosarcoma: A Case Report. Front Vet Sci. 9:847933.
Flesner BK, Wood GW, Gayheart-Walsten P, et al. 2020. Autologous cancer cell vaccination, adoptive T-cell transfer, and interleukin-2 administration results in long-term survival for companion dogs with osteosarcoma. J Vet Intern Med. 34:2056-2067.
Fletcher CDM, Bridge JA, Hogendoorn PCW, et al. 2013. WHO Classification of Tumours of Soft Tissue and Bone. 4th ed, Vol 5. Geneva (CH): WHO Press.
Franchini D, Paci S, Ciccarelli S, et al. 2022. Chondroblastic Osteosarcoma Associated with Previous Chronic Osteomyelitis Caused by Serratia liquefactions in a German Shepherd Dog. Vet Sci. 9:36.
Franklin RT, Aronson E, Fallon RK, et al. 1985. Femoral osteomyelitis and osteosarcoma in a dog. Vet Radiol. 26:211-213.
Fritz SE, Henson MS, Greengard E, et al. 2016. A phase I clinical study to evaluate safety of orally administered, genetically engineered Salmonella enterica serovar Typhimurium for canine osteosarcoma. Vet Med Sci. 2:179-190.
Galindo-Zamora V, von Babo V, Eberle N, et al. 2016. Kinetic, kinematic, magnetic resonance and owner evaluation of dogs before and after the amputation of a hind limb. BMC Vet Res. 12:20.
Gamblin RM, Straw RC, Powers BE, et al. 1995. Primary osteosarcoma distal to the antebrachiocarpal and tarsocrural joints in nine dogs (1980-1992). J Am Anim Hosp Assoc. 31:86-91.
Garzotto CK, Berg J, Hoffmann WE, et al. 2000. Prognostic significance of serum alkaline phosphatase activity in canine appendicular osteosarcoma. J Vet Intern Med. 14:587-592.
Gasch EG, Rivier P, Bardet JF. 2013. Free proximal cortical ulnar autograft for the treatment of distal radial osteosarcoma in a dog. Can Vet J. 54:162-6.
Gearing DP, Virtue ER, Gearing RP, et al. 2013. A fully caninised anti-NGF monoclonal antibody for pain relief in dogs. BMC Vet Res 9:226.
Gibbs CP, VG Kukekov, Reith JD, et al. 2005. Stem-like cells in bone sarcomas: Implications for Tumorigenesis. Neoplasia 7:967-976.
Gillett NA, Hahn FF, Mewhinney JA, et al. 1985. Osteosarcoma development following single inhalation exposure to americium-241 in beagle dogs. Radiat Res 104:83-93.
Gillette SM, Gillette EL, Powers BE, et al. 1990. Radiation-induced osteosarcoma in dogs after external beam or intraoperative radiation therapy. Cancer Res 50:54-57.
Gilley RS, Hiebert E, Clapp K, et al. 2017. Long-term formation of aggressive bony lesions in dogs with mid-diaphyseal fractures stabilized with metallic plates: incidence in a tertiary referral hospital population. Front Vet Sci 4:3.
Gillings SL, Johnson J, Fulmer A, et al. 2009. Effect of a 1‐hour IV infusion of doxorubicin on the development of cardiotoxicity in dogs as evaluated by electrocardiography and echocardiography. Vet Ther. 10:46‐58.
Gold R, Oliveira F, Poll R. 2018. Zygomatic Arch Parosteal Osteosarcoma in Dogs and a Cat. Vet Pathol. 56:274-276.
Gorman E, Barger AM, Wypij JM et al. 2006. Cutaneous metastasis of primary appendicular osteosarcoma in a dog. Vet Clin Pathol 35:358–361.
Green EM, Adams WM, Forrest LJ. 2002. Four fraction palliative radiotherapy for osteosarcoma in 24 dogs. J Am Anim Hosp Assoc. 38:445-451.
Hall JL, Mason SL. 2019. Limb sparing achieved by ray amputation for osteosarcoma of the left third metacarpal bone in a Labrador. Vet Rec Case Rep. 7: e000752.
Hallman BE, Hauck ML, Williams LE, et al. 2019. Incidence and risk factors associated with development of clinical cardiotoxicity in dogs receiving doxorubicin. J Vet Intern Med. 33:783-791.
Hanafy E, Al Jabri A, Gadelkarim G, et al. 2018. Tumor histopathological response to neoadjuvant chemotherapy in childhood solid malignancies: is it still impressive? J Investig Med. 66:289-297.
Hans EC, Pinard C, van Nimwegen SA, et al. 2018. Effect of surgical site infection on survival after limb amputation in the curative-intent treatment of canine appendicular osteosarcoma: a Veterinary Society of Surgical Oncology retrospective study. Vet Surg. 47:E88-E96.
Hartmann JT, Knop S, Fels LM, et al. 2000. The use of reduced doses of amifostine to ameliorate nephrotoxicity of cisplatin/ifosfamide-based chemotherapy in patients with solid tumors. Anticancer Drugs. 11:1-6.
Henry CJ, Brewer WG Jr, Whitley EM, et al. 2005. Veterinary Cooperative Oncology Group (VCOG). Canine digital tumors: a veterinary cooperative oncology group retrospective study of 64 dogs. J Vet Intern Med. 19:720-4.
Hernigou P, Thiery JP, Benoit J, et al. 1989. Methotrexate diffusion from acrylic cement. Local chemotherapy for bone tumours. J Bone Joint Surg. 71-B:804-811.
Hillers KR, Dernell WS, Lafferty MH, et al. 2005. Incidence and prognostic importance of lymph node metastases in dogs with appendicular osteosarcoma: 228 cases (1986–2003) J Am Vet Med Assoc. 226:1364–1367.
Holmberg BJ, Farese JP, Taylor D, et al. 2004. Osteosarcoma of the humeral head associated with osteochondritis dissecans in a dog. J Am Anim Hosp Assoc. 40:246-249.
Hosoya K, Poulson JM, Azuma C. 2008. Osteoradionecrosis and radiation induced bone tumors following orthovoltage radiation therapy in dogs. Vet Radiol Ultrasound 49:189-195.
Huff J, Lunn RM, Waalkes MP, et al. 2007. Cadmium-induced Cancers in Animals and in Humans. Int J Occup Environ Health. 13:202-212.
Ilizarov GA. 1992. The tension-stress effect on the genesis and growth of tissues. In: The Transosseous osteosynthesis. Berlin, Germany: Springer-Verlag, 137-255.
Imran H, Enders F, Krailo M, et al. 2009. Effect of time to resumption of chemotherapy after definitive surgery on prognosis for non-metastatic osteosarcoma. J Bone Joint Surg Am. 91:604–612.
Isaka M, Kokubo D, Sakai T. 2021. The occurrence of osteosarcoma after tibial fracture repair in a dog. Open Vet J. 11:11-13.
Jehn CT, Lewis DD, Farese JP, et al. 2007. Transverse ulnar bone transport osteogenesis: a new technique for limb salvage for the treatment of distal radial osteosarcoma in dogs. Vet Surg. 36:324-34.
Jeys LM, Grimer RJ, Carter SR, et al. 2007. Post operative infection and increased survival in osteosarcoma patients: are they associated? Ann Surg Oncol. 14:2887-95.
Jimenez IA, Pool RR, Fischetti AJ, et al. 2022. Neoplastic transformation of arteriopathy-derived bone infarct into nascent osteosarcoma in the proximal tibia of a miniature schnauzer. Vet Rec Case Rep e293.
Jimenez IAJ, Pool RR, Gabrielson KL. 2022. Canine Idiopathic Arteriopathy, Appendicular Bone Infarcts, and Neoplastic Transformation of Bone Infarcts in 108 Dogs (Canis lupus familiaris). Comp Med. 72:306-319.
Jones SA, Gilmour LJ, Ruoff CM, et al. 2020. Radiographic features of histologically benign bone infarcts and bone infarcts associated with neoplasia in dogs. J Am Vet Med Assoc 256:1352-1358.
Karlsson EK, Sigurdsson S, Ivansson E, et al. 2013. Genome-wide analyses implicate 33 loci in heritable dog osteosarcoma, including regulatory variants near CDKN2A/B. Genome Biol. 14:R132.
Ketring AR. 1987. 153-Sm-EDTMP and 186-Re-HEDP as bone therapeutic radiopharmaceutical. International Journal of Radiation Applications and Instrumentation. Part B. Nuc Med Biol. 14:223-232.
Kirkpatrick CJ, Alves A, Köhler H, et al. 2000. Biomaterial-induced sarcoma: a novel model to study preneoplastic change. Am J Pathol. 156:1455-1467.
Kirpensteijn J, Kik M, Rutterman GR, et al. 2002. Prognostic significance of a new histologic grading system for canine osteosarcoma. Vet Pathol 39:240-246.
Kirpensteijn J, Steinheimer D, Park RD, et al. 1998. Comparison of cemented and non-cemented allografts in dogs with osteosarcoma. Vet Comp Orthop Traumatol 11:178–184.
Kirpensteijn J, Teske E, Kik M, et al. 2002. Lobaplatin as an adjuvant chemotherapy to surgery in canine appendicular osteosarcoma: a phase II evaluation. Anticancer Res. 22:2765-70.
Kirpensteijn J, Van Den Bos R, Endenburg N. 1999. Adaptation of dogs to the amputation of a limb and their owners’ satisfaction with the procedure. Vet Rec. 144:115-118.
Knapp-Hoch HM, Fidel JL, Sellon RK, et al. 2009. An expedited palliative radiation protocol for lytic or proliferative lesions of appendicular bone in dogs. J Am Anim Hosp Assoc. 45:24-32.
Krimins RA, Fritz J, Gainsburg LA, et al. 2017. Use of magnetic resonance imaging-guided biopsy of a vertebral body mass to diagnose osteosarcoma in a rottweiler. J Am Vet Med Assoc. 250:779-784
Kuntz CA, Asselin TL, Dernell WS, et al. 1998. Limb salvage surgery for osteosarcoma of the proximal humerus: outcome in 17 dogs. Vet Surg. 27:417-22.
LaRue SM, Withrow SJ, Powers BE, et al. 1989. Limb-sparing treatment for osteosarcoma in dogs. J Am Vet Med Assoc 195:1734-1744.
Lascelles BDX, Dernell WS, Correa MT, et al. 2005. Improved survival associated with postoperative wound infection in dogs treated with limb-salvage surgery for osteosarcoma. Ann Surg Oncol. 12:1073–1083.
Lattimer JC, Corwin LA, Stapleton J, et al. 1990. Clinical and Clinicopathologic Response of Canine Bone Tumor Patients to Treatment with Samarium-153-EDTMP. J Nucl Med. 31:1316-1325.
LeBlanc AK, Mazcko CN, Cherukuri A, et al. 2021. Adjuvant Sirolimus Does Not Improve Outcome in Pet Dogs Receiving Standard-of-Care Therapy for Appendicular Osteosarcoma: A Prospective, Randomized Trial of 324 Dogs. Clin Cancer Res. 27:3005-3016.
Leibman NF, Kuntz CA, Steyn PF, et al. 2001. Accuracy of radiography, nuclear scintigraphy, and histopathology for determining the proximal extent of distal radial osteosarcoma in dogs. Vet Surg 30:240–245.
Levine AM, Rosenberg SA. 1979. Alkaline phosphatase level in osteosarcoma tissue are related to prognosis. Cancer. 44:2291-2293.
Liptak JM, Dernell WS, Ehrhart N, et al. 2006. Cortical allograft and endoprosthesis for limb-sparing surgery in dogs with distal radial osteosarcoma: a prospective clinical comparison of two different limb-sparing techniques. Vet Surg. 35:518-33.
Liptak JM, Dernell WS, Ehrhart N, et al. 2006. Cortical allograft and endoprosthesis for limb-sparing surgery in dogs with distal radial osteosarcoma: a prospective clinical comparison of two different limb-sparing techniques. Vet Surg. 35:518–533.
Liptak JM, Dernell WS, Ehrhart N, et al. 2004. Canine Appendicular Osteosarcoma: Curative-Intent Treatment. Compend Contin Educ Pract Vet, 26:186-197, 2004.
Liptak JM, Dernell WS, Lascelles BDX, et al. I2004. ntraoperative extracorporeal irradiation for limb sparing in 13 dogs. Vet Surg. 33:446–456.
Liptak JM, Dernell WS, Lascelles BDX, et al. 2001. Survival analysis of dogs with appendicular osteosarcoma treated with limb sparing surgery and adjuvant carboplatin or carboplatin and doxorubicin. Proc 18th Annu Vet Cancer Soc Conf 21:39.
Liptak JM, Ehrhart N, Santoni BG, et al. 2006. Cortical bone graft and endoprosthesis in the distal radius of dogs: A biomechanical comparison of two different limb-sparing techniques. Vet Surg. 35:150–160.
Liptak JM, Pluhar GE, Dernell WS, et al. 2005. Limb-sparing surgery in a dog with osteosarcoma of the proximal femur. Vet Surg. 34:71-7.
Loukopoulos P, Robinson WF. 2007. Clinicopathologic relevance of tumour grading in canine osteosarcoma. J Comp Pathol 136:65-73.
MacDonald TL, Schiller TD. 2010. Limb-sparing surgery using tantalum metal endoprosthesis in a dog with osteosarcoma of the distal radius. Can Vet J. 51:497-500.
Makielski KM, Mills LJ, Sarver AL, et al. 2019. Risk factors for development of canine and human osteosarcoma: a comparative review. Vet Sci 6:48.
Marino DJ, Matthiesen DT, Stefanacci JD, et al. 1995. Evaluation of dogs with digit masses: 117 cases (1981-1991). J Am Vet Med Assoc. 207:726-728.
Martin TW, Griffin L, Custis J, et al. 2021. Outcome and prognosis for canine appendicular osteosarcoma treatment with stereotactic body radiation therapy in 123 dogs. Vet Comp Oncol. 19:284-294.
Mantyh PW. 2014. Bone cancer pain: from mechanism to therapy. Curr Opin Support Palliat Care. 8:83–90.
Marcellin-Little DJ, DeYoung DJ, Thrall DE, et al. 1999. Osteosarcoma at the site of bone infarction associated with total hip arthroplasty in a dog. Vet Surg 28:54-60.
Marconato L, Buracco P, Polton GA, et al. 2021. Timing of adjuvant chemotherapy after limb amputation and effect on outcome in dogs with appendicular osteosarcoma without distant metastases. J Am Vet Med Assoc. 259:749-756.
Mason NJ, Gnanandarajah JS, Engiles JB, et al. 2016. Immunotherapy with a HER2-Targeting Listeria Induces HER2-Specific Immunity and Demonstrates Potential Therapeutic Effects in a Phase I Trial in Canine Osteosarcoma. Clin Cancer Res. 22:4380-90.
Mayer MN & Grier CK. 2006. Palliative radiation therapy for canine osteosarcoma. Can Vet J. 47:707-709.
McNeill CJ, Overley B, Shofer FS, et al. 2007. Characterization of the biological behaviour of appendicular osteosarcoma in Rottweilers and a comparison with other breeds: a review of 258 dogs. Vet Comp Oncol. 5:90–98.
Mehl ML, Seguin B, Dernell WS, et al. 2005. Survival analysis of one versus two treatments of local delivery cisplatin in a biodegradable polymer for canine osteosarcoma. Vet Comp Oncol. 3:81-6.
Meyers PA, Heller G, Healey J, et al. 1992. Chemotherapy for nonmetastatic osteogenic sarcoma: the Memorial Sloan-Kettering experience. J Clin Oncol 10:5–15.
Milner RJ, Dormehl I, Louw WK, et al. 1998. Targeted radiotherapy with Sm-153-EDTMP in nine cases of canine primary bone tumours. J S Afr Vet Assoc. 69:12–17.
Mitchell KE, Boston SE, Kung M, et al. 2016. Outcomes of Limb-Sparing Surgery Using Two Generations of Metal Endoprosthesis in 45 Dogs With Distal Radial Osteosarcoma. A Veterinary Society of Surgical Oncology Retrospective Study. Vet Surg. 45:36-43.
Modiano JF, Bellgrau D, Cutter GR, et al. 2012. Inflammation, apoptosis, and necrosis induced by neoadjuvant fas ligand gene therapy improves survival of dogs with spontaneous bone cancer. Mol Ther. 20:2234-43.
Monteiro BP, de Lorimier LP, Moreau M, et al. 2018. Pain characterization and response to palliative care in dogs with naturally-occurring appendicular osteosarcoma: An open label clinical trial. PLoS One. 13:e0207200.
Morello E, Buracco P, Martano M, et al. 2001. Bone allografts and adjuvant cisplatin for the treatment of canine appendicular osteosarcoma in 18 dogs. J Small Anim Pract 42:61–66.
Morello E, Vasconi E, Martano M, et al. 2003. Pasteurized tumoral autograft and adjuvant chemotherapy for the treatment of canine distal radial osteosarcoma: 13 cases. Vet Surg. 32:539-544.
Muir P, Ruaux-Mason CP. 2000. Microcrack density and length in the proximal and distal metaphases of the humerus and radius in dogs. Am J Vet Res. 61:6-8.
Murphy ST, Parker RB, Woodard JC. 1997. Osteosarcoma following total hip arthroplasty in a dog. J Small Anim Pract 38:263–267.
Musser ML, Berger EP, Parsons C, et al. 2019. Vaccine strain Listeria monocytogenes abscess in a dog: a case report. BMC Vet Res.15:467.
Musser ML, Berger EP, Tripp CD, et al. 2021. Safety evaluation of the canine osteosarcoma vaccine, live Listeria vector. Vet Comp Oncol. 19:92-98.
Nagamine E, Hirayama K, Matsuda K, et al. 2015. Diversity of histologic patterns and expression of cytoskeletal proteins in canine skeletal osteosarcoma. Vet Pathol 52:977-984.
NIH National Cancer Institute. Disease information: Osteosarcoma (https://ccr.cancer.gov/comparative-oncology-program/disease-info)
Norquest CJ, Maitz CA, Keys DA, et al. 2022. Fracture rate and time to fracture in dogs with appendicular osteosarcoma receiving finely fractionated compared to coarsely fractionated radiation therapy: A single institution study. Vet Med Sci. 8:1013-1024.
Ogilvie GK, Straw RC, Jameson VJ, et al. 1993. Evaluation of single- agent chemotherapy for treatment of clinically evident osteosarcoma metastases in dogs: 45 cases (1987–1991). J Am Vet Med Assoc 202:304–306.
Ogilvie GK, Straw RC, Jameson VJ, et al. 1993. Evaluation of single-agent chemotherapy for treatment of clinically evident osteosarcoma metastases in dogs: 45 cases (1987-1991). J Am Vet Med Assoc. 202:304-6.
Olsen EJ, Carlson CS. 2012. Bones, joints, tendons, and ligaments. In: Zachary JF, ed. Pathologic Basis of Veterinary Disease. 6th ed. St. Louis, MO: Elsevier; 954–1009.
Owen LN, Bostock DE. 1971. Multiple cartilaginous exostoses with development of a metastasizing osteosarcoma in a Shetland Sheepdog. J Small Animal Pract. 12:507-512.
Pagano C, Boudreaux B, Shiomitsu K. 2016. Safety and toxicity of an accelerated coarsely fractionated radiation protocol for treatment of appendicular osteosarcoma in 14 dogs: 10 Gy x 2 fractions. Vet Radiol Ultrasound. 57:551-556.
Paoloni MC, Mazcko C, Fox E, et al. 2010. Rapamycin pharmacokinetic and pharmacodynamic relationships in osteosarcoma: a comparative oncology study in dogs. PLoS One. 5:e11013.
Pluhar GE. 2016. Fracture-Associated Sarcoma. In: Complications in Small Animal Surgery, First Edition.
Poon AC, Matsuyama A, Mutsaers AJ. 2020. Recent and current clinical trials in canine appendicular osteosarcoma. Can Vet J. 61:301-308.
Pooya HA, Séguin B, Mason DR, et al. 2004. Biomechanical comparison of cortical radial graft versus ulnar transposition graft limb-sparing techniques for the distal radial site in dogs. Vet Surg. 33:301–308.
Powers BE, Withrow SJ, Thrall DE, et al. 1991. Percent tumor necrosis as a predictor of treatment response in canine osteosarcoma. Cancer. 67:126-134.
Prior C, Watrous BJ, Penfold D. 1985. Radial diaphyseal osteosarcoma with associated bone infarctions in a dog. J Am Anim Hosp Assoc 22:43-48.
Pull SS, Yazdi AR, Ghert M, et al. 2021. Bone cement as a local chemotherapeutic drug delivery carrier in orthopedic oncology: a review. J Bone Oncol. 26.
Ramirez O 3rd, Dodge RK, Page RL, et al. 1999. Palliative radiotherapy of appendicular osteosarcoma in 95 dogs. Vet Radiol Ultrasound. 40:517-22.
Richardson SM, Wurtz LD, Collier CD. 2022. Ninety Percent or Greater Tumor Necrosis Is Associated With Survival and Social Determinants of Health in Patients With Osteosarcoma in the National Cancer Database. Clin Orthop Relat Res.
Ringdahl-Maryland B, Thamm DH, Martin TW. 2022. Retrospective evaluation of outcome in dogs with appendicular osteosarcoma following hypo fractionated palliative radiation therapy with or without bisphosphonates: 165 cases (2010-2019). Front Vet Sci.
Riser WH, Brodey RS, Biery DN. 1972. Bone infarctions associated with malignant bone tumors in dogs. J Am Vet Med Assoc 160:414-421.
Risselada M, Tuohy JL, Law M, et al. 2020. Local Administration of Carboplatin in Poloxamer 407 After an Ulnar Osteosarcoma Removal in a Dog. J Am Anim Hosp Assoc. 56:325.
Rovesti GL, Bascucci M, Schmidt K, et al. 2002.. Limb sparing using a double bone-transport technique for treatment of a distal tibial osteosarcoma in a dog. Vet Surg 31:70-77.
Ru G, Terracini B, Glickman LT. 1998. Host related factors for canine osteosarcoma. Vet J 156:31-39.
Rubin JA, Suran JN, Brown DC, et al. 2015. Factors associated with pathological fractures in dogs with appendicular primary bone neoplasia: 84 cases (2007–2013). J Am Vet Med Assoc 247:917–923.
Sabattini S, Renzi A, Buracco P, et al. 2017. Comparative assessment of the accuracy of cytological and histologic biopsies in the diagnosis of canine bone lesions. J Vet Intern Med. 31:864-871.
Sakthikumar S, Elvers I, Kim J, et al. 2018. SETD2 Is Recurrently Mutated in Whole-Exome Sequenced Canine Osteosarcoma. Cancer Res. 78:3421–3431.
Salyer SA, Wavreille VA, Fenger JM, et al. 2020. Evaluation of microwave ablation for local treatment of dogs with distal radial osteosarcoma: A pilot study. Vet Surg. 49:1396-1405.
Sapierzynski R, Czopowicz M. 2017. The animal-dependent risk factors in canine osteosarcomas. Pol J Vet Sci. 20:293–298.
Schajowicz F, Sissons HA, Sobin LH. 1995. The World Health Organization’s histologic classification of bone tumors. A commentary on the second edition. Cancer. 75:1208–1214.
Schott CR, Tatiersky LJ, Foster RA, et al. 2018. Histologic grade does not predict outcome in dogs with appendicular osteosarcoma receiving the standard of care. Vet Pathol 55:202-212.
Séguin B, O'Donnell MD, Walsh PJ, et al. 2017. Long-term outcome of dogs treated with ulnar rollover transposition for limb-sparing of distal radial osteosarcoma: 27 limbs in 26 dogs. Vet Surg. 46:1017-1024.
Séguin B, Walsh PJ, Mason DR, et al. 2003. Use of an ipsilateral vascularized ulnar transposition autograft for limb-sparing surgery of the distal radius in dogs: An anatomic and clinical study. Vet Surg 32:69-79.
Selmic LE, Burton JH, Thamm DH, et al. 2014. Comparison of carboplatin and doxorubicin-based chemotherapy protocols in 470 dogs after amputation for treatment of appendicular osteosarcoma. J Vet Intern Med. 28:554-63.
Selmic LE, Ryan SD, Boston SE, et al. 2014. Osteosarcoma following tibial plateau leveling osteotomy in dogs: 29 cases (1997-2011). J Am Vet Med Assoc. 244:1053-1059.
Selmic LE, Ryan SD, Ruple A, et al. 2018. Association of tibial plateau leveling osteotomy with proximal tibial osteosarcoma in dogs. J Am Vet Med Assoc. 253:752-756.
Sharma S, Boston SE, Riddle D, et al. 2020. Osteosarcoma of the proximal tibia in a dog 6 years after tibial tuberosity advancement. Can Vet J. 61:946-950.
Shor S, Fadl-Alla BA, Pondenis HC, et al. 2015. Expression of nociceptive ligands in canine osteosarcoma. J Vet Intern Med. 29:268-75.
Simpson S, Dunning MD, de Brot S, et al. 2017. Comparative review of human and canine osteosarcoma: morphology, epidemiology, prognosis, treatment, and genetics. Acta Vet Scand 59:71.
Sivacolundhu RK, Runge JJ, Donovan TA, et al. 2013. Ulnar osteosar- coma in dogs: 30 cases (1992-2008). J Am Vet Med Assoc. 243:96-101.
Skorupski KA, Uhl JM, Szivek A, et al. 2016. Carboplatin versus alternating carboplatin and doxorubicin for the adjuvant treatment of canine appendicular osteosarcoma: a randomized, phase III trial. Vet Comp Oncol. 14:81-7.
Slayter MV, Boosinger TR, Pool RR, et al. 1994. World Health Organization. International histologic classification of tumors of domestic animals, histological classification of bone and joint tumors of domestic animals. Washington, DC: Armed Forces Institute of Pathology, American Registry of Pathology.
Spodnick GJ, Berg J, Rand WM, et al. 1992. Prognosis for dogs with appendicular osteosarcoma treated by amputation alone: 162 cases (1978–1988). J Am Vet Med Assoc 200:995–999.
Sternberg RA, Pondenis HC, Yang X, et al. 2013. Association between absolute tumor burden and serum bone-specific alkaline phosphatase in canine appendicular osteosarcoma. J Vet Intern Med. 27:955-63.
Stevenson S, Hohn RB, Pohler OE, et al. 1982. Fracture-associated sarcoma in the dog. J Am Vet Med Assoc. 180:1189-1196.
Straw RC, Withrow SJ. 1996. Limb-sparing surgery versus amputation for dogs with bone tumors. Vet Clin North Am Small Anim Pract 26:135–143.
Straw RC, Withrow SJ, Douple EB, et al. 1994. Effects of cis-diam-minedichloroplatinum II released from D,L-polylactic acid implanted adjacent to cortical allografts in dogs. J Orthop Res. 12:871–7.
Straw RC, Withrow SJ, Powers BE. 1990. Management of canine appendicular osteosarcoma. Vet Clin North Am Small Anim 20:1141-1161.
Straw RC, Withrow SJ, Richter SL, et al. 1991. Amputation and cisplatin for treatment of canine osteosarcoma. J Vet Intern Med. 5:205-10.
Tang N, Song WX, Luo J, et al. 2008. Osteosarcoma development and stem cell differentiation. Clin Orthop Relat Res 466:2114-2130.
Thompson KG, Dittmer KE. 2017. Tumors of bone. In: Meuten DJ, ed. Tumors in Domestic Animals. 5th ed. Ames, IA: John Wiley & sons, Inc; 356–424.
Thompson JP, Fugent MJ. 1992. Evaluation of survival times after limb amputation, with and without subsequent administration of cisplatin, for treatment of appendicular osteosarcoma in dogs: 30 cases (1979-1990). J Am Vet Med Assoc. 200:531-3.
Thrall DE. 2018. Radiographic features of bone tumors and bone infections in dogs and cats. Textbook of veterinary diagnostic radiology. Elsevier; 390-402.
Tommasini Degna M, Ehrhart N, Feretti A, et al. 2000. Bone transport osteogenesis for limb salvage following resection of primary bone tumors: Experience with six cases (1991-1996). Vet Comp Orthop Traumatol 13:18-22.
Tripp CD, Fidel J, Anderson CL, et al. 2011. Tolerability of metronomic administration of lomustine in dogs with cancer. J Vet Intern Med. 25:278-84.
Tuohy JL, Shaevitz MH, Garrett LD, et al. 2019. Demographic characteristics, site and phylogenetic distribution of dogs with appendicular osteosarcoma: 744 dogs (2000-2015). PLoS One. 14:e0223243.
Unni KK, Dahlin DC, Beabout JW, et al. 1976. Parosteal osteogenic sarcoma. Cancer. 37:2466–2475.
UyBico SJ, Motamedi K, Omura MC, et al. 2012. Relevance of compartmental anatomical guidelines for biopsy of musculoskeletal tumors: retrospective review of 363 biopsies over a 6-year period. J Vasc Interview Radio. 23:511-518.
Wallack ST, Wisner ER, Werner JA, et al. 2002. Accuracy of magnetic resonance imaging for estimating intramedullary osteosarcoma extent in preoperative planning of canine limb-salvage procedures. Vet Radiol Ultrasound 43:432– 441.
Walsh P, Dassler C, Preston C, et al. 2000. The use of a vascularized cortical autograft to limb spare dogs with distal radial osteosarcoma. Proc 18th Annu Vet Cancer Soc Conf 20:62.
Withrow SJ, Liptak JM, Straw RC, et al. 2004. Biodegradable cisplatin polymer in limb-sparing surgery for canine osteosarcoma. Ann Surg Oncol. 11:705-13.
Withrow SJ, Powers BE, Straw RC, et al. 1991. Comparative aspects of osteosarcoma. Dog versus man. Clin Orthop Relat Res 270:159-168.
Welch RD, Lewis DD. 1999. Distraction osteogenesis. Vet Clin North Am Small Anim Pract 29:1187-1205.
Wouda RM, Luu SW, Roush JK, et al. 2018. Bilateral osteosarcoma associated with metallic implant sites in two dogs. Israel J Vet Med. 73:39-44.
Yasumasu T, Ueda T, Uozumi J, et al. 1992. Comparative study of cisplatin and carboplatin on pharmacokinetics, nephrotoxicity and effect on renal nuclear DNA synthesis in rats. Pharmacol Toxicol. 70:143-7.


